TGF-β Inhibitors Clinical Trial Pipeline Accelerates as 25+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
The TGF-β inhibitors market is gaining momentum due to their ability to address significant unmet needs in oncology and fibrosis, where existing therapies often show limited effectiveness. The market is expected to surge with the launch of therapies such as AdAPT-001, SRK-181, STP 707, SIS 201 CD, IOA-359, Elritercept, ISTH0036, TU-2218, AGMB-129, IO170, ES 014, SHR-1701, HEC585, RO7204239, ESN-Y, and others.
New York, USA, Sept. 23, 2025 (GLOBE NEWSWIRE) -- TGF-β Inhibitors Clinical Trial Pipeline Accelerates as 25+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
The TGF-β inhibitors market is gaining momentum due to their ability to address significant unmet needs in oncology and fibrosis, where existing therapies often show limited effectiveness. The market is expected to surge with the launch of therapies such as AdAPT-001, SRK-181, STP 707, SIS 201 CD, IOA-359, Elritercept, ISTH0036, TU-2218, AGMB-129, IO170, ES 014, SHR-1701, HEC585, RO7204239, ESN-Y, and others.
DelveInsight’s 'TGF-β Inhibitors Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for TGF-β inhibitors across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the TGF-β inhibitors domain.
Key Takeaways from the TGF-β Inhibitors Pipeline Report
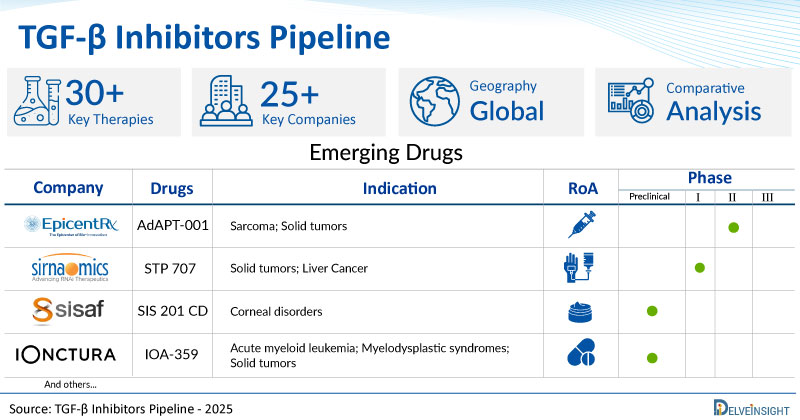
- DelveInsight’s TGF-β inhibitors pipeline report depicts a robust space with 25+ active players working to develop 30+ pipeline TGF-β inhibitors.
- Key TGF-β inhibitor companies, such as EpicentRx, Scholar Rock, Sirnaomics, SiSaf, iOnctura, Keros Therapeutics, Isarna Therapeutics, TiumBio, Agomab Therapeutics, IO Biotech, Elpiscience Biopharmaceuticals, Jiangsu Hengrui Medicine, Sunshine Lake Pharma, Roche, Enveda Biosciences, and others, are evaluating new TGF-β inhibitor drugs to improve the treatment landscape.
- Promising pipeline TGF-β inhibitors such as AdAPT-001, SRK-181, STP 707, SIS 201 CD, IOA-359, Elritercept, ISTH0036, TU-2218, AGMB-129, IO170, ES 014, SHR-1701, HEC585, RO7204239, ESN-Y, and others are under different phases of TGF-β inhibitors clinical trials.
- In July 2025, Keros Therapeutics announced that the first patient was dosed in the Phase III RENEW clinical trial of elritercept in adults with transfusion-dependent anemia with very low, low, or intermediate risk myelodysplastic syndromes (“MDS”). The dosing of the first patient triggers a USD 10 million milestone payment to Keros under the global license agreement with Takeda.
- In May 2025, Isarna Therapeutics shared final positive results from the Phase II BETTER trial, which evaluated the candidate ISTH0036 to address retinal fibrosis. ISTH0036 is an investigational antisense oligonucleotide designed to selectively suppress the production of transforming growth factor beta 2 (TGF-β2), a key cytokine involved in fibrosis and disease progression in retinal pathologies.
- In May 2025, updated efficacy data of TU-2218, which is being developed by TiumBio, from a Phase I/II trial in solid tumours was presented at the 61st Annual Meeting of the American Society of Clinical Oncology (ASCO 2025).
- In May 2025, Agomab Therapeutics announced late-breaking interim data from the ongoing STENOVA1 Phase IIa clinical trial for AGMB-129, an oral gastrointestinal (GI)-restricted small molecule inhibitor of ALK5 (TGF-b RI or ALK5) developed for the potential treatment of Fibrostenosing Crohn’s Disease (FSCD). The interim results were presented by Florian Rieder, MD, at Digestive Disease Week® (DDW) 2025, which took place in San Diego on May 3-6, 2025..
- In February 2025, IO Biotech announced new preclinical findings at the AACR-IO conference. Their T-β-directed vaccine IO170 significantly reduced tumor growth in models of pancreatic adenocarcinoma and prostate cancer, reshaping the tumor microenvironment without systemic toxicity. The company is preparing for an FDA IND submission.
- In December 2024, EpicentRx announced that the US Food and Drug Administration (FDA) granted Fast Track designation for the oncolytic adenovirus-delivered transforming growth factor beta (TGFβ) inhibitor, AdAPT-001, plus the anti-PD-1, nivolumab, or anti-PD-L1, atezolizumab, to treat recurrent or refractory advanced or metastatic soft tissue sarcoma (STS) with disease progression after at least one prior line of therapy.
- In October 2024, TiumBio Co., Ltd., a clinical-stage biopharmaceutical company focused on discovering and developing innovative therapeutics for patients with rare and incurable diseases, announced that the first patient has been dosed in its Phase II clinical trial of TU2218.
Request a sample and discover the recent advances in TGF-β inhibitor drugs @ TGF-β Inhibitors Pipeline Report
The TGF-β inhibitors pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage TGF-β inhibitors drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the TGF-β inhibitors clinical trial landscape.
TGF-β Inhibitors Overview
Transforming growth factor (TGF)-β is a secreted cytokine with diverse biological functions. It exerts its effects through TGF-β type I and II receptors on the plasma membrane and intracellular SMAD transcription factors. Dysregulated TGF-β signaling, both inside and outside the cell, plays a critical role in cancer progression. In healthy tissues and during the early stages of tumor development, TGF-β acts as a tumor suppressor by inducing epithelial growth arrest. However, in advanced cancers, once the cytostatic influence of TGF-β is bypassed, its signaling shifts toward tumor promotion by driving epithelial-to-mesenchymal transition (EMT), stimulating angiogenesis, and enabling immune evasion. Consequently, targeting the TGF-β pathway has emerged as a promising strategy for cancer therapy.
Find out more about TGF-β inhibitor drugs @ TGF-β Inhibitors Analysis
TGF-β Inhibitors Competitive Landscape
Leading companies such as EpicentRx, Scholar Rock, Sirnaomics, SiSaf, iOnctura, Keros Therapeutics, Isarna Therapeutics, TiumBio, Agomab Therapeutics, IO Biotech, Elpiscience Biopharmaceuticals, Jiangsu Hengrui Medicine, Sunshine Lake Pharma, Roche, Enveda Biosciences, and others are currently active in the TGF-β inhibitors competitive landscape.
Apart from EpicentRx’s AdAPT-001, which is in phase II for sarcoma and solid tumors, the other TGF-β inhibitors, including Sirnaomics’ STP 707, SiSaf’s SIS 201 CD, iOnctura’s IOA-359, Enveda Biosciences’ ESN-Y, and others, are in early stages of development.
Learn more about the emerging TGF-β inhibitors @ TGF-β Inhibitors Clinical Trials
TGF-β Inhibitors Therapeutics Assessment
The TGF-β inhibitors pipeline report proffers an integral view of the emerging TGF-β inhibitors segmented by stage, product type, molecule type, and route of administration.
Scope of the TGF-β Inhibitors Pipeline Report
- Coverage: Global
- TGF-β Inhibitors Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- TGF-β Inhibitors Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- TGF-β Inhibitors Therapeutics Assessment By Route of Administration: Infusion, Intradermal, Intramuscular, Intranasal, Oral, Parenteral, Subcutaneous, Topical
- TGF-β Inhibitors Therapeutics Assessment By Molecule Type: Gene therapies, Small molecule, Vaccines, Polymers, Peptides, Monoclonal antibodies
- Key TGF-β Inhibitors Companies: EpicentRx, Scholar Rock, Sirnaomics, SiSaf, iOnctura, Keros Therapeutics, Isarna Therapeutics, TiumBio, Agomab Therapeutics, IO Biotech, Elpiscience Biopharmaceuticals, Jiangsu Hengrui Medicine, Sunshine Lake Pharma, Roche, Enveda Biosciences, and others
- Key Pipeline TGF-β Inhibitors: AdAPT-001, SRK-181, STP 707, SIS 201 CD, IOA-359, Elritercept, ISTH0036, TU-2218, AGMB-129, IO170, ES 014, SHR-1701, HEC585, RO7204239, ESN-Y, and others
Dive deep into rich insights for new TGF-β inhibitors, visit @ TGF-β Inhibitors Drugs
Table of Contents
| 1. | TGF-β Inhibitors Pipeline Report Introduction |
| 2. | TGF-β Inhibitors Pipeline Report Executive Summary |
| 3. | TGF-β Inhibitors Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | TGF-β Inhibitors Clinical Trial Therapeutics |
| 6. | TGF-β Inhibitors Pipeline: Late-Stage Products (Pre-registration) |
| 7. | TGF-β Inhibitors Pipeline: Late-Stage Products (Phase III) |
| 8. | TGF-β Inhibitors Pipeline: Mid-Stage Products (Phase II) |
| 9. | TGF-β Inhibitors Pipeline: Early-Stage Products (Phase I) |
| 10. | TGF-β Inhibitors Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the TGF-β Inhibitors Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the TGF-β Inhibitors Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the TGF-β inhibitors pipeline therapeutics, reach out @ TGF-β Inhibitors Therapeutics
Related Reports
TGF Inhibitors Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key TGF inhibitors companies, including Bristol Myers Squibb, Sanofi, Kintor Pharma, Scholar Rock, Oncotelic, Corbus Pharmaceuticals, and iOnctura, among others.
TGF-Beta-1 Inhibitor Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key TGF-beta-1 inhibitor companies, including MedPacto, EpicentRx, Sirnaomics, Scholar Rock, SiSaf, Isarna Therapeutics, among others.
TGF-Beta-2 Inhibitor Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key TGF-beta-2 inhibitor companies, including Bristol Myers Squibb, Merck (MSD), Jiangsu Hengrui Pharma, Eli Lilly, Roche, Gilead, Sanofi, Novartis, and Kintor Pharma, among others.
Glioblastoma Market Insight, Epidemiology And Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key glioblastoma companies, including Bayer, Chimerix, Aivita Biomedical, Denovo Biopharma, Northwest Therapeutics, VBL Therapeutics, Laminar Pharmaceuticals, MedImmune, DNAtrix, Immunomic Therapeutics, Imvax, MimiVax, CNS Pharmaceuticals, Epitopoietic Research Corporation (ERC), Istari Oncology, SonALAsense, Kintara Therapeutics, Bristol Myers Squibb, Medicenna Therapeutics, BioMimetix, Eisai and Merck Sharp & Dohme, Kazia Therapeutics, Oblato, Genenta Science, Enterome, Inovio Pharmaceuticals, Karyopharm Therapeutics, Forma Therapeutics, VBI Vaccines, TME Pharma, among others.
Non-small Cell Lung Cancer Market
Non-small Cell Lung Cancer Market Insight, Epidemiology And Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key NSCLC companies, including AstraZeneca, Boehringer Ingelheim, Pfizer, Takeda, Johnson & Johnson Innovative Medicine, Eli Lilly and Company, Merck, Bristol-Myers Squibb, Roche, Shanghai Henlius Biotech, AbbVie, Daiichi Sankyo, Nuvation Bio, PDC*line Pharma, Moderna Therapeutics, Pfizer, GSK, Gilead Sciences, BieGene, Nuvalent, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
